Sterically Hindered Phenolic Antioxidants
Some of the most widely used antioxidants are sterically hindered phenols. These compounds act as a primary antioxidants by converting peroxyl radicals to hydroperoxides. Thus, they inhibit autooxidation of organic polymers by reducing the amount of peroxyl radicals. The mechanism of scavenging peroxy radicals is shown below for butylated hydroxytoluene (2,6-di-tert-butyl-4-methylphenol, BHT):
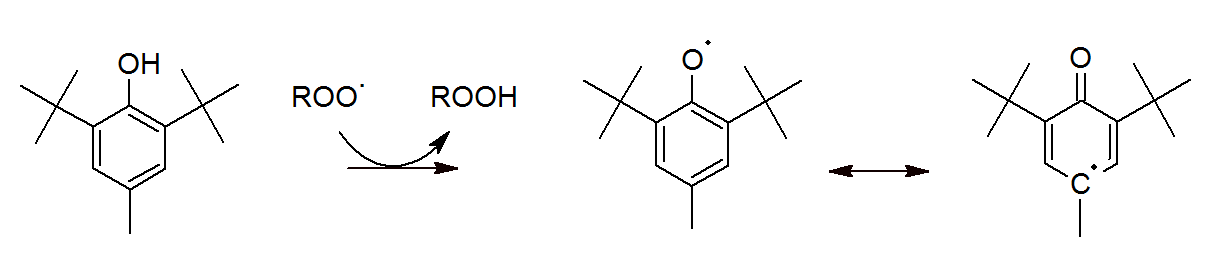
The peroxyl radicals abstract the reactive hydrogen of the BHT. The resulting oxytoluene radicals are stabilized by electron delocalization and by the bulky substituents (steric hindrance) which greatly reduces their reactivity and thus prevents them from reacting with polymer chains (hydrogen abstraction). Instead, the oxytoluene radicals undergo a number of other reactions. For example, they can undergo a bimolecular reaction that produces another BHT molecule and a p-quinomethane or the oxytoluene radical can undergo an irreversible reaction with a second peroxyl radical which thermally decomposes to para-quinone:1
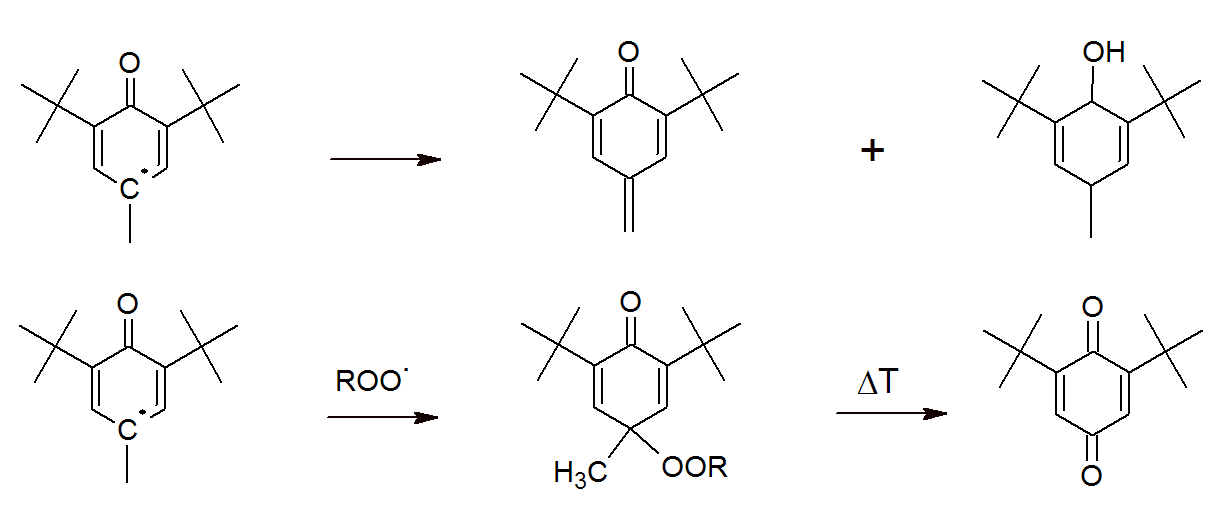
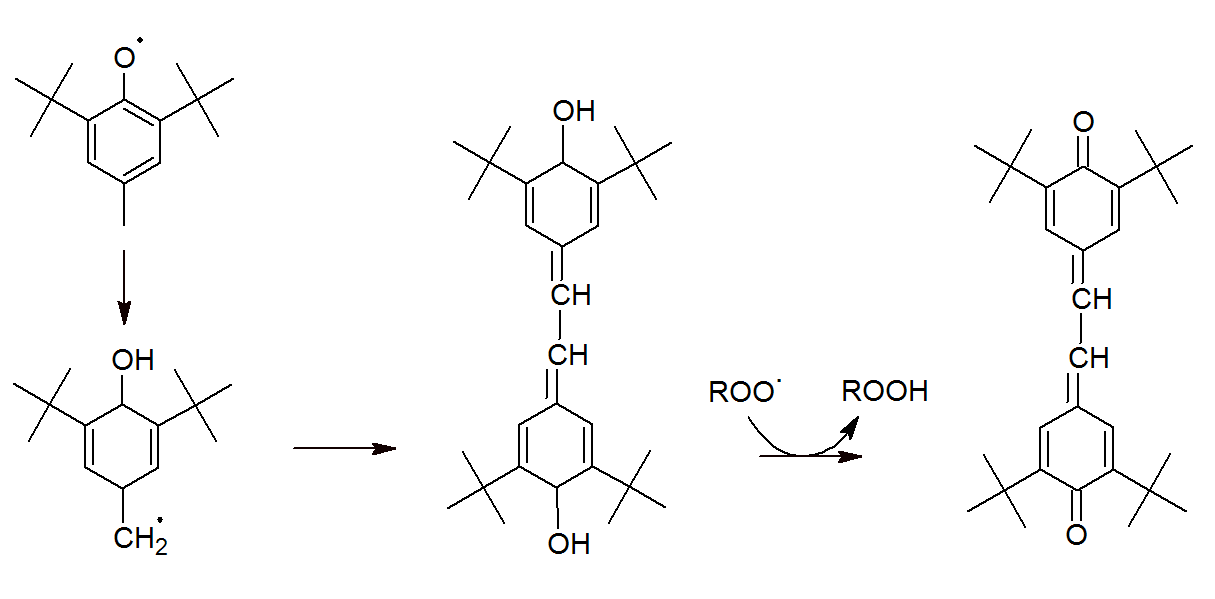
Thus, each BHT molecule can react with two peroxyl radicals to yield products that are very stable to further reactions. Recombination of two oxytoluene radicals is also possible (see Figure below). Thus, all oxytoluene radicals are ultimately transformed to quinonoid structures.
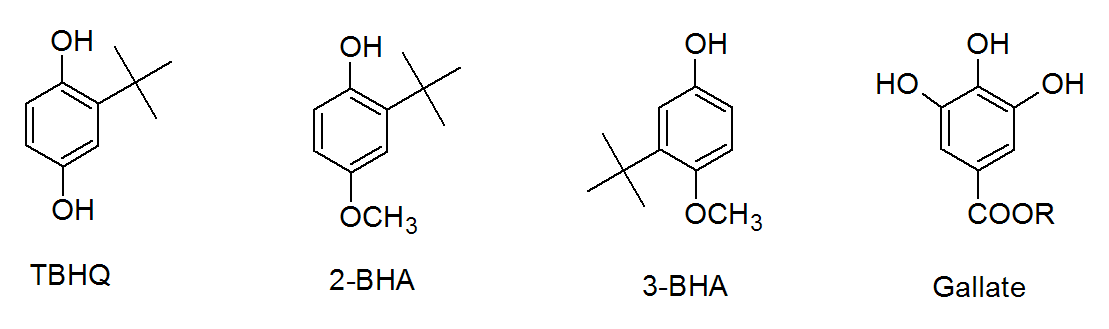
Butylated hydroxytoluene (BHT) is only one among many sterically hindered phenols. Other represenatives include butylated hydroxyanisole (BHA), tertiary-butylhydroquinone (TBHQ) and gallates among many others which all undergo similar reactions.
References
- F. Shahidi, P.P. Janitha, P.D. Wanasundara, Food Science and Nutrition, 32(1), 67-103 (1992)